Add Initial Required Information - EudraCT Number and National Competent Authority details
Task topic including all steps necessary for adding the number and the National Competent Authority (in the case of a planned Clinical Trial) to the system.
Prerequisites for creating a
To create a Clinical Trial Application or add Clinical Trial Information, the following prerequisites apply:
For those in possession of a and data ready for creating a Clinical Trial Application draft, or Third Country Clinical Trial Information, follow these steps:
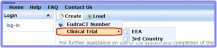
- In the Welcome page click
 and select 'Clinical Trial' and then 'EEA' or 'Third Country' dependent on whether the EudraCT Number you are using is associated with an EEA Clinical Trial Application or a Third Country Clinical Trial Information.
and select 'Clinical Trial' and then 'EEA' or 'Third Country' dependent on whether the EudraCT Number you are using is associated with an EEA Clinical Trial Application or a Third Country Clinical Trial Information.
- In the Initial Required Information screen, click the drop-down field arrow and press the first letter of the National Competent Authority concerned by the Clinical Trial until the desired is highlighted. Press Enter to select.
- Insert the EudraCT Number for the Clinical Trial Application in the free text field (it may be cut and pasted). Note that each Clinical Trial is linked to a particular EudraCT number and may be used for one CTA only.
Note: If you do not have a EudraCT Number,
click
and select

from the top menu bar . See
Create a EudraCT Number section for more details.
- Finally, click
 and the Clinical Trial Application Menu appears with the CTA Information for the draft CTA and the associated NCA displayed clearly on the left-hand side of the screen.
and the Clinical Trial Application Menu appears with the CTA Information for the draft CTA and the associated NCA displayed clearly on the left-hand side of the screen.
See "Clinical Trial Application Menu Overview" for more detailed information on the process of creating a CTA.
Related Topics
 and select 'Clinical Trial' and then 'EEA' or 'Third Country' dependent on whether the EudraCT Number you are using is associated with an EEA Clinical Trial Application or a Third Country Clinical Trial Information.
and select 'Clinical Trial' and then 'EEA' or 'Third Country' dependent on whether the EudraCT Number you are using is associated with an EEA Clinical Trial Application or a Third Country Clinical Trial Information. and select
and select  from the top menu bar . See Create a EudraCT Number section for more details.
from the top menu bar . See Create a EudraCT Number section for more details.  and the Clinical Trial Application Menu appears with the CTA Information for the draft CTA and the associated NCA displayed clearly on the left-hand side of the screen.
and the Clinical Trial Application Menu appears with the CTA Information for the draft CTA and the associated NCA displayed clearly on the left-hand side of the screen.