Create a EudraCT Number
Below are the steps necessary to get a number.
Users require access to the EudraCT application.
A workstation/computer running a web browser (Internet Explorer 7, or above, Firefox, etc.).
In order to complete the creation of a Clinical Trial Application, users must first apply for a .
- Enter the name of the organisation the EudraCT number request is for in the 'Requestor's organisation name' field. This is likely to be the organisation you work for.
- Enter the town/city in which the organisation's office is located in the 'Requestor's organisation town/city' field. This should relate to the office where the particular planned Clinical Trial is to be run from (rather than the global headquarters, for example).
- Use the drop-down to select the country in which the organisation's office, as specified above, is located.
Once the drop-down is open, you can press the first letter of the country on your keyboard to cycle through the possible results.
- Insert the 's Code Number. This is the Protocol Code Number for the Clinical Trial that will be linked to the EudraCT number obtained from this request. It should be entered in the format as specified by your organisation and must be completed.
It is not generated by the EudraCT system. If you are unsure what this value is, contact the Sponsor of the Clinical Trial.
- In the 'Requestor name' field, enter your first name. This is a mandatory field.
- In the 'Requestor last name' field, enter your last name (family name). This is a mandatory field.
- Enter the e-mail address where the should be sent. Any valid e-mail address is acceptable and need not be the requester’s e-mail. This is a mandatory field.
- For security reasons, enter the letters and numbers displayed in the window next to the text field. This entry is case sensitive and mandatory. If you cannot read the text in the picture, click the
 button to refresh the security image.
button to refresh the security image.
- If the EudraCT Number is to be used for a Clinical Trial contained in a Paediatric Investigation Plan (), click the 'Yes' radio button, otherwise click the 'No' radio button.
- If the EudraCT Number is to be used for a Clinical Trial conducted in a third country (outside of the /), click the 'Yes' radio button, otherwise click the 'No' radio button.
- Choose the country within the European Economic Area where the Clinical Trial is currently expected to take place.
Click inside the field list then press the first letter of the country on your keyboard to cycle through the possible results.
To select multiple countries, hold CTRL and click each country in turn. Selected countries are highlighted light blue.
Once the country(ies) is selected, click the 'Copy' button.
- Finally, click
 button.
button.
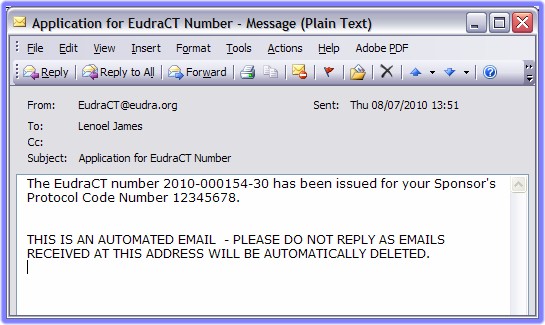
The is generated and appears on the web page. It is also sent to the specified email address, simultaneously.
Click the
button to return to the home page and create a Clinical Trial Application:
An email should appear in the inbox associated with the email address previously specified (step 7, above) with content similar to the following example:
Duplicate Sponsor Protocol Code Number
The EudraCT system will check that the Sponsor’s Protocol Code Number submitted on the form is unique within the EudraCT database. It is very unlikely that different sponsors will use the same Protocol Code Numbers for their trials. However, it may be that the submitted Sponsor’s Protocol Code Number already exists, in which case the following warning message appears when the ‘Get EudraCT Number’ link is taken.
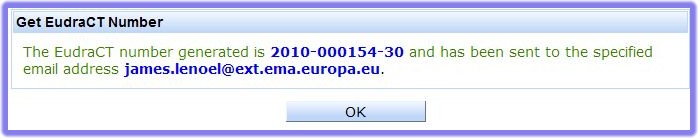
If different sponsors do use the same Protocol Code Numbers for their trials, then within the Community it is the EudraCT number that provides the truly unique reference to the sponsor’s trial. If the system detects a duplicate sponsor protocol code number, the system will warn the requestor and in this case the most likely explanation is that a EudraCT number has already been requested by someone from the same organisation or another collaborator in the trial. The option is given to exit the system without creating a new EudraCT number so that checks within the organisation or trial collaborators can be completed.
N.B. It is recommended that Requestors should exit the system and check for a previously issued EudraCT number before continuing.
See Clinical Trial Application Menu Overview for more detailed information.
 button to refresh the security image.
button to refresh the security image.
 button.
button.

 button to refresh the security image.
button to refresh the security image.
 button.
button.
 button to return to the home page and create a Clinical Trial Application:
button to return to the home page and create a Clinical Trial Application:

