Add a new Substance
Task including steps for requesting the addition of a substance which is not already present in the eXtended EudraVigilance Medicinal Product Dictionary () by the .
This step should be taken only after performing a thorough search for the substance using the 'Add '  on the 'D. Identification' screen to find and add an active substance from the list of available active substances in the eXtended EudraVigilance Medical Product Dictionary ():
on the 'D. Identification' screen to find and add an active substance from the list of available active substances in the eXtended EudraVigilance Medical Product Dictionary ():
For more information, see "To Add an Active Substance(s) to this IMP"
Note:: Users are reminded that registering their organisation with EudraVigilance is the preferred approach for adding new substances to the , see
EV How To Register pages.
The process satisfies the different types of substance which may not already be in and can be divided broadly into the two following groups:
Substance previously part of a Medicinal Product, an IMP with , or backed by bibliographic reference source
- Select the first bullet of the 'Substance details>Substance category'.
Substance under development
- Select the second bullet of the 'Substance details>Substance category'.
Prerequisites for requesting addition of a new substance by :
The following prerequisites apply:
- Users have performed a thorough search of the for the active substance.
- Users have checked that the or agent working on behalf of the Sponsor do not have a EudraVigilance registration allowing direct entry of substances to XEVMPD.
- Users possess the contact details, and substance details for the substance in question before commencing the registration process.
- Click in the 'Name' free text field and include a contact name, should EMA require it.
Note: Mandatory field.
- Click in the 'Telephone number' free text field and include the contact phone number (including country code) of the above named individual, should EMA require it.
Note: Mandatory field.
- Click in the free text field and include the contact email address of the individual/department, should EMA require it.
Note: Mandatory field.
- Click the radio button next to the statement which best describes the active substance being requested for addition.
If the substance is part of an authorised product, then full details of the substance should already be available in the . Click the relevant heading below to see full explanation for completion of the rest of the section:
Insert a name of a substance which is either part of a product used as an IMP for which a Marketing Authorisation is granted, or that is publicly available in a bibliographic reference source (e.g. published in the INN list).
Selecting the first radio button alongside the above heading text provides a further six fields that should be completed by the Sponsor or their agent:
- Click in the 'Substance name' free text field and include the substance name.
Note: Mandatory field.
- Use the 'Substance source' drop-down list to select the relevant option from the substance source list in the .
Note: Mandatory field.
- Select the relevant option from the substance class list in using the 'Substance class' drop-down.
Note: Mandatory field.
- Click in the 'Chemical/Biological description' free text field and include the 'Chemical/Biological Description'. It should be added in its entirety to ensure is able to register the substance correctly.
Note: Optional field. 20000 character limit (including spaces)
- Click 'Select the Document path...' to open a file select dialogue and attach a document to the submission. It should be the Package Insert of the Approved Substance (also known as Patient Information Leaflet).
Note: Only one (optional) attachment is allowed in a , DOC or DOCX format. Files must not exceed 3 MB.
- Click in the 'Enter the characters shown' free text field and enter the characters shown in the blue box.Click 'New Image' to refresh the image, whose letters you should type into the field to the left.
Note: Mandatory field.
- Click
 to submit your request for the addition of the substance, in this single instance, by the EMA.
to submit your request for the addition of the substance, in this single instance, by the EMA.
Note: Only one registration request per Sponsor or agent is allowable..
Insert a name of a substance which is either not part of a product for which a Marketing Authorisation is granted, or that is not publicly available in a bibliographic reference source.
Selecting the second radio button alongside the above heading text provides fields that should be completed by the Sponsor or their agent:
- Click in the 'Substance name' free text field and include the substance name. Alternatively, click in the 'Substance code' field and include your organisation's substance code (e.g. its working or development codename).
Note: It is mandatory to complete one or other field.
- Select the relevant option from the substance class list in using the 'Substance class' drop-down.
Note: Mandatory field.
- Next, select the 'Organisation category' from the three available options. Click the radio button alongside the option that best reflects the type of organisation making the submission. The choice of organisation category must be based on the company or organisation's main business, rather than its role in requesting the addition of a new substance.
Note: Mandatory field.
- Click in the 'Organisation name' free text field and include the full name of the institution or organisation registering the substance.
Note: Mandatory field.
- Click in the 'Provisional organisation identifier' free text field and include your provisional EudraVigilance () Organisation ID. The EV Organisation ID must conform to the following specification:
- A string formed by upper-case letters (A to Z), a number set (0 to 9) or a combination of both.
- Special or country-based characters and blank spaces are not allowed.
- The Organisation ID must consist of a minimum of 3 and a maximum of 10 characters (e.g. PHARMACO23).
- See EudraVigilance for more detailed guidance.
Note: Mandatory field.
- Click in the 'Street address' free text field and add the building name and/or number and street name. Carriage returns may be used within the address field.
Note: Mandatory field.
- Click in the 'City' free text field and include the town or city where the sponsor is based.
Note: Mandatory field.
- Click in the free text field and include the post code (where applicable).
Note: This field is not mandatory. However, if a post code exists, please include it.
- Select the relevant country from the 'Country' drop-down list.
Note: Mandatory field.
- Now, include the personal details of the pharmacovigilance qualified/responsible person to be contacted should further information be required by the EudraVigilance team. Click in each free field and include, 'Title', 'First name' (also known as forename or given name), 'Family name' (also known as surname or last name).
Note: Mandatory fields.
- Click in 'Department' and include the full name of the department of the previously named institution or organisation that the pharmacovigilance qualified/responsible person works in.
Note: Mandatory fields.
- Click in the 'Street address' free text field and add the building name and/or number and street name where the department named above is located. Carriage returns may be used within the address field.
Note: Mandatory field.
- Click in the 'City' free text field and include the town or city where the building named directly above is located.
Note: Mandatory field.
- Click in the free text field and include the post code of the address directly above(where applicable).
Note: This field is not mandatory. However, if a post code exists, please include it.
- Click in the 'Telephone number' free text field and include the land line telephone number of the pharmacovigilance qualified/responsible person named above. Do the same for the 'Mobile telephone number' and 'Fax number, if you have this information
Note: It is mandatory to include one telephone number.
- Should you be a third party acting on behalf of the sponsor, then select the 'Yes' radio button beside the question "Are you a third party acting on behalf of the Sponsor?" complete the 'Third party provider details' below. Click 'No' if you are the sponsor and proceed to step 18.
Note: Mandatory field.
- If you are a third party provider acting on behalf of the Sponsor, complete the 'Third party provider details', which will differ from those included in the 'Qualified/Responsible Person' contact section above. The same guidance may be used for completing these fields.
Note: Mandatory field.
- Click 'Select the Document path...' to open a file select dialogue and attach a document to the submission. An attachment is a mandatory requirement and must be either the relevant part of the investigator's brochure OR the clinical trial protocol.
Note: Mandatory. One document is allowed in a PDF, DOC or DOCX format. Files must not exceed 3 MB.
- Click in the 'Enter the characters shown' free text field and enter the characters shown in the blue box.Click 'New Image' to refresh the CAPTCHA image, whose letters you should type into the field to the left.
Note: Mandatory field.
- Click
 to submit your request for the addition of the substance, in this single instance, by the EMA.
to submit your request for the addition of the substance, in this single instance, by the EMA.
Note: Only one registration request per Sponsor or agent is allowable..
D. MPD Add Active Substance Success
If the registration passes the validation process, the success message screen appears, outlining the steps that will be taken upon EMA's receipt of the substance registration:
Now go to the "Clinical Trial Application Menu Overview" for more detailed information of other functionality.
Related Topics
 on the 'D. IMPAcronym: Investigational Medicinal Product. A pharmaceutical form of an active substance or placebo being tested or used as a reference in a Clinical Trial, including products already with a marketing authorisation but used or assembled in a different way from the authorised form, or when used for an unauthorised indication, or when used to gain further information about the authorised form. Identification' screen to find and add an active substance from the list of available active substances in the eXtended EudraVigilance Medical Product Dictionary (XEVMPDeXtended EudraVigilance Medicinal Product Dictionary.):
on the 'D. IMPAcronym: Investigational Medicinal Product. A pharmaceutical form of an active substance or placebo being tested or used as a reference in a Clinical Trial, including products already with a marketing authorisation but used or assembled in a different way from the authorised form, or when used for an unauthorised indication, or when used to gain further information about the authorised form. Identification' screen to find and add an active substance from the list of available active substances in the eXtended EudraVigilance Medical Product Dictionary (XEVMPDeXtended EudraVigilance Medicinal Product Dictionary.):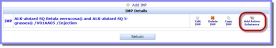
 to submit your request for the addition of the substance, in this single instance, by the EMA.
to submit your request for the addition of the substance, in this single instance, by the EMA.