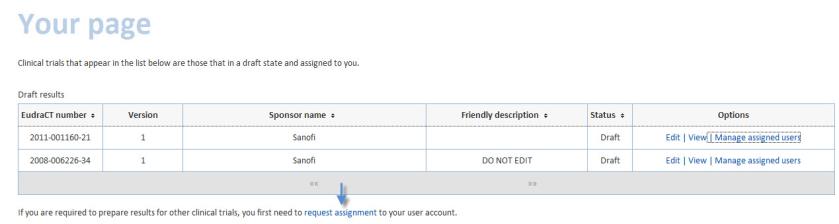
Your page is an overview of clinical trials which have been assigned to a results user.
From this page you can edit, view the results that are in draft and view users assigned to the clinical trial.Some users are also able to delegate new users and manage the assigned users. Additionally, you can request an assignment to other clinical trials.
This page also allows you view posted and finalised results for clinical trials assigned to you.

To request assignment to one or more additional clinical trials, click the request assignment link:
Enter each EudraCTEudraCT (European Union Drug Regulating Authorities Clinical Trials) is the European Clinical Trials Database of all interventional clinical trials of medicinal products commencing in the European Union from 1 May 2004 onwards. The EudraCT database has been established in accordance with Directive 2001/20/EC. number on a new line:
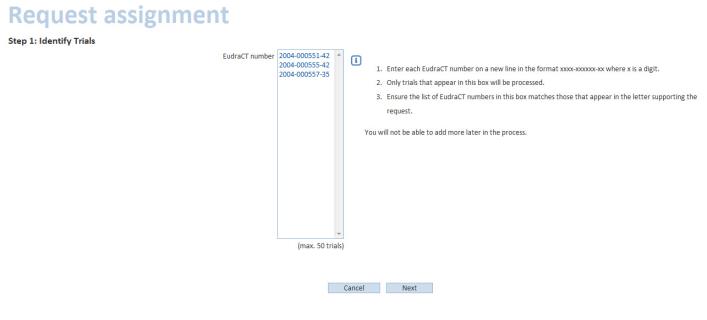
Click Next and enter the full name of the trial and the name of the sponsor organisations (s):
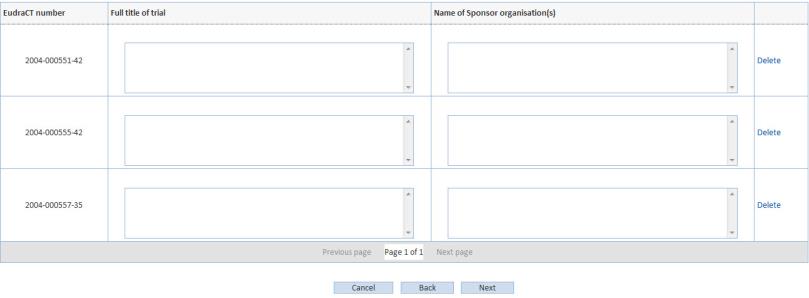
Click Next. Select Attach authorising letter and make sure all EudraCT numbers entered in this request are also mentioned in the attached letter.
All EudraCT numbers mentioned in the letter must be included in this request, or they will not be considered part of the request. The attachment must be a scanned image containing the required hand-written signature.
Please note:Only PDF, BMP, JPG, GIF and PNG formats are permitted. Also note the maximum file size for an attachment is 5 MB
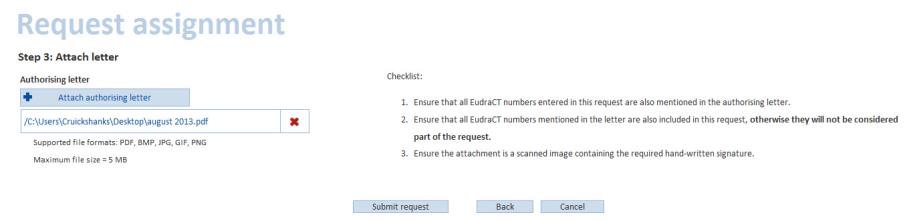
When the document is attached, click Submit request.A confirmation is displayed confirming receipt:
Click Done to return to the your results home page.
Related Topics: