Task topic including steps necessary for validating a Clinical Trial Application draft.
The system includes very few data consistency checks whilst the CTAcronym: Clinical Trial - A Clinical Trial Application becomes a Clinical Trial and is searchable within EudraCT once approved. Application form is being created. The application now allows users to validate the Clinical Trial Application based on predefined business rules.
These business rules may be reviewed online in the EudraCTEudraCT (European Union Drug Regulating Authorities Clinical Trials) is the European Clinical Trials Database of all interventional clinical trials of medicinal products commencing in the European Union from 1 May 2004 onwards. The EudraCT database has been established in accordance with Directive 2001/20/EC. page.
• Click on the ‘EudraCT Validation Rules’ link in the User Guides section of the page.


Now go to the Review Validate Application Results section below for more detailed information on reviewing and understanding the Validate Application Results.
This sub-section is intended to provide information on reviewing the Validate Application Validation Results screen: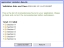
 at the bottom of the screen to save the entire report as a PDFAcronym: Portable Document Format (PDF) is an open standard for document exchange. The file format created by Adobe Systems in 1993 is used for representing two-dimensional documents in a manner independent of the application software, hardware, and operating system. document or click
at the bottom of the screen to save the entire report as a PDFAcronym: Portable Document Format (PDF) is an open standard for document exchange. The file format created by Adobe Systems in 1993 is used for representing two-dimensional documents in a manner independent of the application software, hardware, and operating system. document or click  to go back to the Clinical Trial Application Menu.
to go back to the Clinical Trial Application Menu.
 to go back to the Clinical Trial Application Menu.
to go back to the Clinical Trial Application Menu.Now go to the "Clinical Trial Application Menu Overview" for more detailed information on other tasks that can be performed with your Clinical Trial Application.