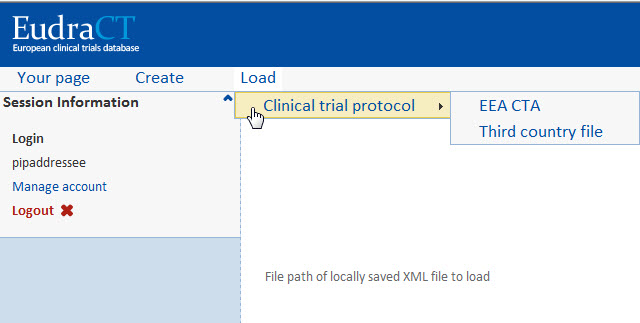
As part of preparing results for a clinical trial, users can upload an XMLeXtensible Markup Language file containing results data that has been prepared by a back-office system or manually amended.

To upload an XML file click Load>Clinical trial protocol and select either EEAEuropean Economic Area. Created in 1994, the EEA combines the countries of the European Union and member countries of EFTA (European Trade Association) Countries that belong to the EEA are: Austria, Belgium, Bulgaria, Czech Republic, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom. Countries that are EEA member countries but NOT part of the European Union are: Norway, Iceland, and Liechtenstein. CTAClinical Trial Application or a Third country file:
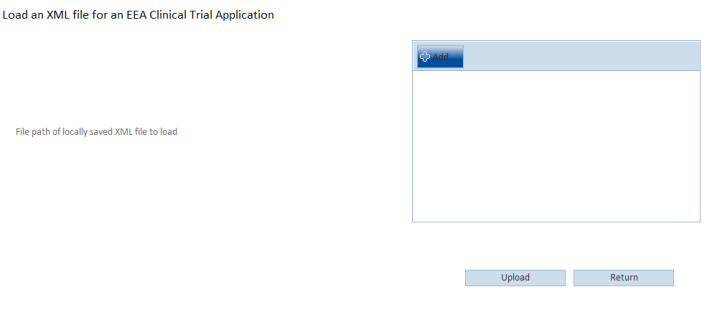
Click Add to locate the file and Upload to load it.

To remove the file click Clear.
Related Topics: