Save XML
In general this saving as an should be done following the complete application or third country file.
Note: If the data is saved to an XML file and you exit the system without saving the full application or third country data, any new data in the application, is lost.
To save a or third country file as an XML file, the following prerequisites apply:
- A CTA or third country file loaded in the clinical trial application menu.
The clinical trial application menu includes the option Save as XML
- Click to Save as XML:
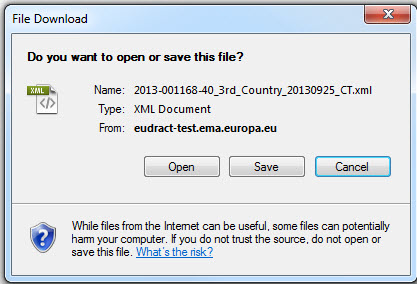
Click Save to save the file locally.
The CTA or third country file, in XML format may now be used either within or outside .
Related Topics:
Save A Clinical Trial Application as XML