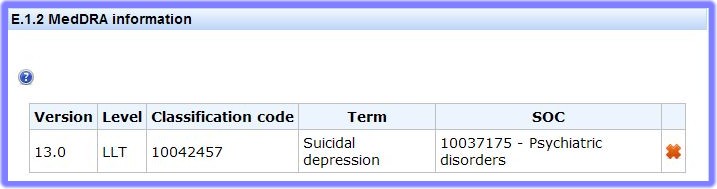
This sub-section covers the design of the Clinical Trial.
This is a MANDATORY section and each sub question should be answered.
- If E.8.1 is 'Yes', E.8.1.1-E.8.1.7.1 applying to the design of the trial should be completed.
Note: In a controlled trial, the tested product is compared to a reference treatment. The reference treatment can be, for example, a placebo, a product known to be effective, a surgical procedure, or a different dose of the same product.
- In E.8.1.1, if each subject in the trial is randomly assigned to receive either the study treatment or a placebo, select 'Yes'.
- In E.8.1.2, if the investigators and the subjects know which treatment is actually given, select 'Yes'.
- In E.8.1.3, if the subjects (healthy volunteers or patients) included in the trial don't know which treatment they are given, select 'Yes'.
- In E.8.1.4, if the investigators and the subjects included in the trial (healthy volunteers or patients) don't know which treatment is given, select 'Yes'.
- In E.8.1.5, select 'Yes', if the trial compares groups of subjects concurrently, each group receiving different dose or treatment.
- In E.8.1.6, select 'Yes', if comparing two (or more) treatments in which patients are switched to the alternative treatment after a specified period of time.
- In E.8.1.7, if there is another methodological characteristic to the trial design, select 'Yes' and complete free text field E.8.1.7.1 with a description.
- In E.8.1.7.1, click in the free text field and include details of the trial design (up to 100 characters). Click the
 to add text in another language than English.
to add text in another language than English.
Add Content in Another Language:
- Click the
 button to add text in another language than English.
button to add text in another language than English.
- Then click
 to select the language you wish to add content in.
to select the language you wish to add content in.
- Click
 if you add a field in error, or if you wish to delete a previously added language translation.
if you add a field in error, or if you wish to delete a previously added language translation.
- In E.8.2.1, select 'Yes' if the comparator drug is another medicinal product.
-
In E.8.2.2, select 'Yes' if the comparator drug is a placebo.
Warning: If the placebo is only used in the trial in order to maintain the blind, the placebo should not be considered as a comparator and 'No' should be selected.
- In E.8.2.3, if the comparator is neither another medicinal product nor a placebo, select 'Yes' here and provide details in the free text field below.
- In E.8.2.3.1, click in the free text field and include details of comparators which are neither other medicinal products nor placebos - e.g. A medical device, a surgical procedure, the lack of treatment, a different treatment schedule, different dosage of the same product (up to 100 characters). Click the
 to add text in another language than English.
to add text in another language than English.
Add Content in Another Language:
- Click the
 button to add text in another language than English.
button to add text in another language than English.
- Then click
 to select the language you wish to add content in.
to select the language you wish to add content in.
- Click
 if you add a field in error, or if you wish to delete a previously added language translation.
if you add a field in error, or if you wish to delete a previously added language translation.
- In E.8.2.4, click in the free text field and include the number of treatment arms (groups) in the trial (up to 10 characters).
Note on Section E.8.2:
In a comparative trial, the investigational product or marketed product is compared against a standard drug (or placebo). The standard (or reference) or placebo medication is called the comparator drug.
Ref: " drug". Pharmaceutical Medicine Dictionary. Philadelphia: Elsevier Health Sciences, 2001. Credo Reference. Web. 26 January 2010.
Warning: If the placebo is not used as a comparator but is only used in the trial in order to maintain the blind, the placebo should not be considered as a comparator and 'No' should be ticked for the item E.8.2.2.
- In E.8.3, select 'Yes' if the trial is conducted in a single centre (clinical trial site) in the Member State concerned by the application.
-
In E.8.4, select 'Yes' if the trial is conducted in multiple sites in the concerned Member State. In this case, the number of sites in the Member State concerned should be entered in section E.8.4.1, below.
- Click in the free text field E.8.4.1 and include the number of sites in the Member State concerned where the trial will take place (up to 2 numbers).
-
In E.8.5, select 'Yes' if the trial will be conducted in more than one Member State of the .
- In E.8.5.1,click in the free text field and include the number of sites in the European Economic Area where the trial is planned to take place (up to 2 numbers).
Note: Please include the sites in the Member State concerned in your total.
- In E.8.6.1, select 'Yes' if the trial involves Sites in at least one Member State and at least one third country. A third country means a country which is not a Member State of the /EEA.
- In E.8.6.2, select 'Yes' if the trial only involves Investigator sites in third country. A third country means a country which is not a Member State of the EU/EEA.
- In E.8.6.3, select the countries if either of the previous two questions were answered 'Yes'. Multiple selection of Options:To select one value, click the value and then click 'Copy'.
To select more than one value, hold CTRL then click the other value you wish to select, then click 'Copy'.
To delete all selected values from the right-hand field, click 'Remove All' button. Note: The above technique can be used for any options organised in the same format of two fields.
- In E.8.6.4, click in the free text field and include the number of sites outside the European Economic Area where the trial is planned to take place (up to 2 numbers).
- In E.8.7, select 'Yes' if an independent data monitoring committee will be used for this trial.
- In E.8.8, click in the free text field and, if it is the last visit of the last subject, enter 'LVLS'. If it is not 'LVLS', provide the definition and justification (up to 500 characters).Click the
 to add text in another language than English.
to add text in another language than English.
Add Content in Another Language:
- Click the
 button to add text in another language than English.
button to add text in another language than English.
- Then click
 to select the language you wish to add content in.
to select the language you wish to add content in.
- Click
 if you add a field in error, or if you wish to delete a previously added language translation.
if you add a field in error, or if you wish to delete a previously added language translation.
- In E.8.9, the duration should be measured from the 1st inclusion until the last visit of the last subject (LVLS).
- In E.8.9.1, click in each field and enter relevant numbers for years, months and days (up to 2 numbers per field).
- In E.8.9.2, click in each field and enter relevant numbers for years, months and days (up to 2 numbers per field).
Note: E.8.9.2 should not be answered if the clinical trial takes places in a single country (i.e. E.8.5 and/or E.8.6.1 are answered 'No'.).
- In E.8.10.1, enter the date on which recruitment of subjects for the trial is planned to commence in the MS concerned in the following format: YYYY-MM-DD.
Tip:Alternatively, click the calendar and select the start date.
- In E.8.10.2,enter the date on which recruitment of subjects for the trial is planned to commence in all countries in the following format: YYYY-MM-DD.
Tip:Alternatively, click the calendar and select the start date.
Note: E.8.10.2 should not be answered if the clinical trial takes places in a single country (i.e. E.8.5 and/or E.8.6.1 are answered 'No'.).
- Click 'Done' and the Clinical Trial Application Menu appears again.
 button to add text in another language than English.
button to add text in another language than English. to select the language you wish to add content in.
to select the language you wish to add content in. if you add a field in error, or if you wish to delete a previously added language translation.
if you add a field in error, or if you wish to delete a previously added language translation. button to add text in another language than English.
button to add text in another language than English. to select the language you wish to add content in.
to select the language you wish to add content in. if you add a field in error, or if you wish to delete a previously added language translation.
if you add a field in error, or if you wish to delete a previously added language translation.