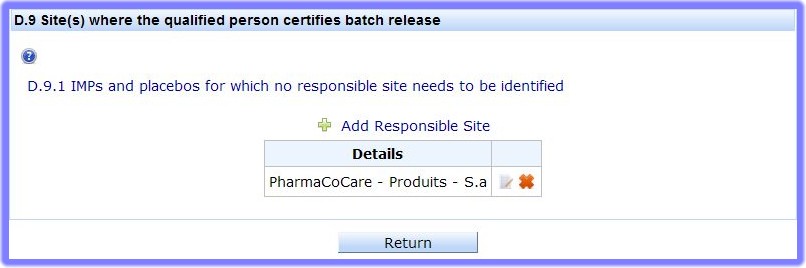
This section is used to identify IMPs and placebos which:
- Have a Marketing AuthorisationThe approval granted by the Regulatory Authority to market a specific product in a particular country. (MAAcronym: Marketing Authorisation) in the EUAcronym: European Union. and
- Are sourced from the EU market and
- Are used in the trial without modification (e.g. not over-encapsulated) and
- The packaging and labelling is carried out for local use only as per article 9.2. of the Directive 2005/28/ECAcronym: European Commission (GCPAcronym: Good Clinical Practice Directive).
- If all the above conditions are met, click the tick box (D.9.2).
- In the 'Finished IMPAcronym: Investigational Medicinal Product. A pharmaceutical form of an active substance or placebo being tested or used as a reference in a Clinical Trial, including products already with a marketing authorisation but used or assembled in a different way from the authorised form, or when used for an unauthorised indication, or when used to gain further information about the authorised form.' table directly beneath D.9.2 select the IMPs and placebos which meet the above criteria.
- Click
 to return to D.9 Site(s) where the qualified person certifies batch release.
to return to D.9 Site(s) where the qualified person certifies batch release.
Now go to the Clinical Trial Application Menu Overview for more detailed information.