
There are several ways that you can interact with the EudraCTEudraCT (European Union Drug Regulating Authorities Clinical Trials) is the European Clinical Trials Database of all interventional clinical trials of medicinal products commencing in the European Union from 1 May 2004 onwards. The EudraCT database has been established in accordance with Directive 2001/20/EC. system:
The session timeout for EudraCT is set to 30 minutes. After 30 minutes of inactivity you are automatically logged out and any changes made are lost unless they have been saved. You will then will need to login in again. To avoid any loss of data we encourage you to save your work often
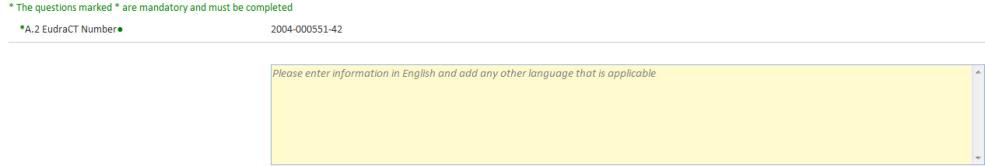
The free text field allows the entry of any text. Some fields may have a limit on the number of characters allowed and is displayed in the top right of the field.

To enter text within the field click in the field or use the tab key to move to the next field.
On each screen, mandatory fields are marked with an asterisk. If the field is a text field, it is shaded yellow.
In result related fields, mandatory fields are marked with a red asterisk.
The green dot shown in a protocol related field indicates that the contents of this field will be public when the clinical trial protocol is published.
Some fields are mandatory only upon the condition of another field being populated in a particular way. For example, if field A is Yes then field B is mandatory. This is a conditionally mandatory field.
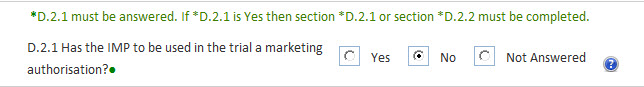
One of a set of radio buttons should be clicked.

A check box is also known as a tick box and when empty is considered unselected.
To select a check box, click within the check box so that a tick appears. Click again to un-tick it and so unselect the field.
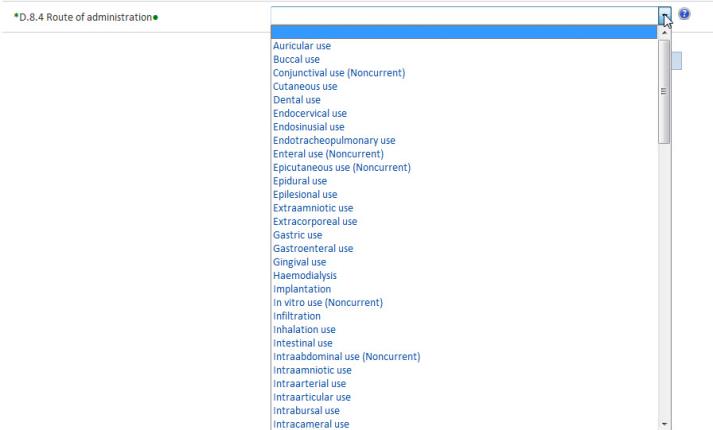
Drop-down lists contain pre-determined items from which a limited number of options to a question may be selected.
To select an item from the drop-down list, click the arrow or the field and the list opens.
To select multiple items click Ctrl hold down the mouse button and select the required items.
The calendar selector icon opens a calendar to ensure accurate selection of dates for entry into the system for input, and for search queries.
Click the calendar icon and the calendar is displayed:
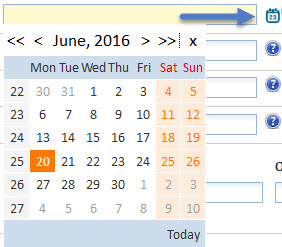
To select a date from the dialogue, click a day. Click the double arrow (<</>>) to move to the previous/next year or the single arrow (</>) to move to the previous/next month.
Once a date has been selected, the calendar closes and the field is populated in the following format: YYYY-MM-DD (e.g. 20th OF June 2016 is represented by 2016-06-20).
The Done button allows you to return to the main index and view the CTAClinical Trial Application sections. If validation errors are displayed, click Done again to remove them and you are returned to the index. This does not allow you to save anything, but allows you to leave one section to move to another section.
The add button, when clicked will expand the section to display additional fields to input:
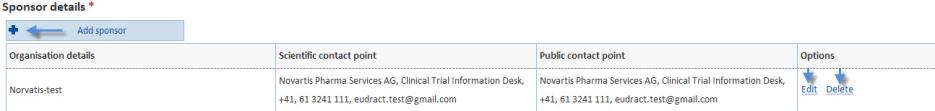
From here you can also Edit information, or Delete it.
Additionally, a drop-down list with the add button, allows you to select an item from a list. Select the required item and click the Add button. The item is added to a list.
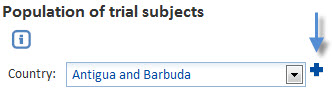
When clicked, this selection is added to the list:
Related Topics: