 button has been selected before inserting the required EudraCT number:
button has been selected before inserting the required EudraCT number:The concept of a mandatory field in the EudraCTEudraCT (European Union Drug Regulating Authorities Clinical Trials) is the European Clinical Trials Database of all interventional clinical trials of medicinal products commencing in the European Union from 1 May 2004 onwards. The EudraCT database has been established in accordance with Directive 2001/20/EC. system, is any field which must be completed before a user can advance the Clinical Trial Application, or Inspection.
If a mandatory field is not completed, an error message appears at the top of the page indicating which field has not been completed.
In this example, the  button has been selected before inserting the required EudraCT number:
button has been selected before inserting the required EudraCT number:
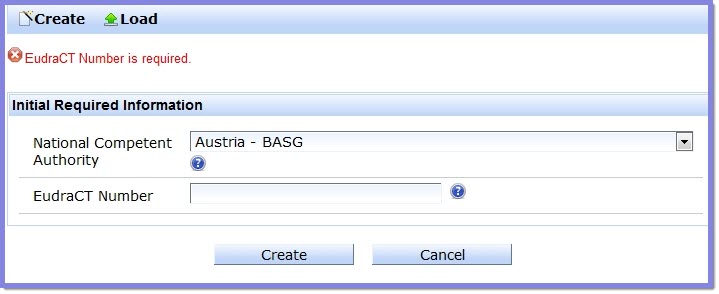
The red text at the top of the screen indicates what mandatory field requires a value.