 , select 'Clinical Trial' then 'EEAAcronym: European Economic Area. Created in 1994, the EEA combines the countries of the European Union and member countries of EFTA (European Trade Association)
Countries that belong to the EEA are: Austria, Belgium, Bulgaria, Czech Republic, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom.
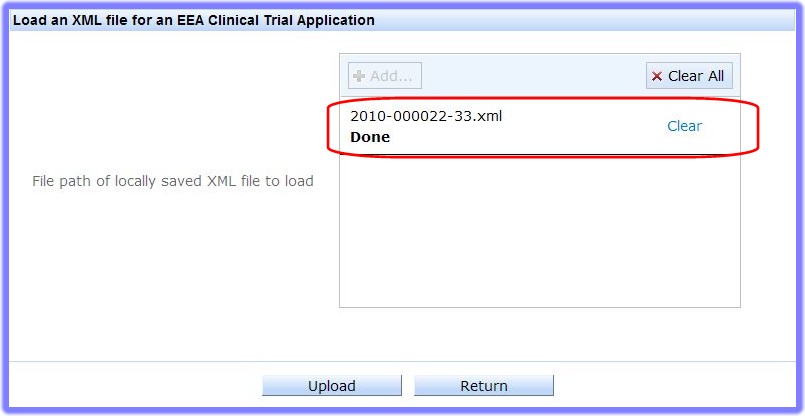
Countries that are EEA member countries but NOT part of the European Union are: Norway, Iceland, and Liechtenstein.' or '3rd Country', depending on the type of XML you wish to load.and the relevant XML Upload screen appears (EEA, in the example):
, select 'Clinical Trial' then 'EEAAcronym: European Economic Area. Created in 1994, the EEA combines the countries of the European Union and member countries of EFTA (European Trade Association)
Countries that belong to the EEA are: Austria, Belgium, Bulgaria, Czech Republic, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom.
Countries that are EEA member countries but NOT part of the European Union are: Norway, Iceland, and Liechtenstein.' or '3rd Country', depending on the type of XML you wish to load.and the relevant XML Upload screen appears (EEA, in the example):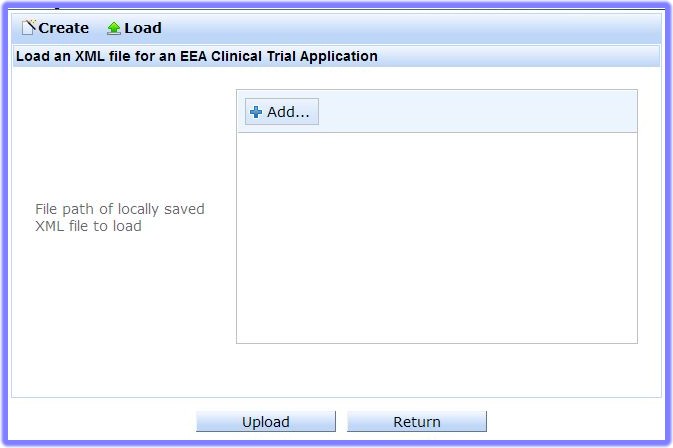
 to open your system browser, and find the file in your workstation's available areas:
to open your system browser, and find the file in your workstation's available areas: 
 .
. .
.