 Compare Clinical Trial Application
Compare Clinical Trial Application
Task topic including steps necessary for comparing a Clinical Trial Application draft with another.
This feature enables users to compare Clinical Trial Application forms on the system (and stored locally) in a way that indicates differences between the two CTAs, much like a track changes function in Microsoft Word. It can be performed with CTAs in all the areas of the database, including those which are being viewed.
Note: Compare functionality is not available for Clinical Trials forms.
Prerequisites for Comparing a :
To compare a CTA with another, the following prerequisites apply:
- A CTA (EEA or Third Country) loaded in in the Clinical Trial Application Menu.
- A locally stored CTA XML of the same type to compare with the loaded CTA.
- All users of may access this functionality if the above prerequisites are met.
- One Clinical Trial Application (or CTA in progress) must be loaded in the system:

- Choose either:
 Clinical Trial Application or
Clinical Trial Application or  so that you advance to the EudraCT Clinical Trial Application Menu.
so that you advance to the EudraCT Clinical Trial Application Menu.
- Once a CTA is loaded, the Clinical Trial Application Menu appears:
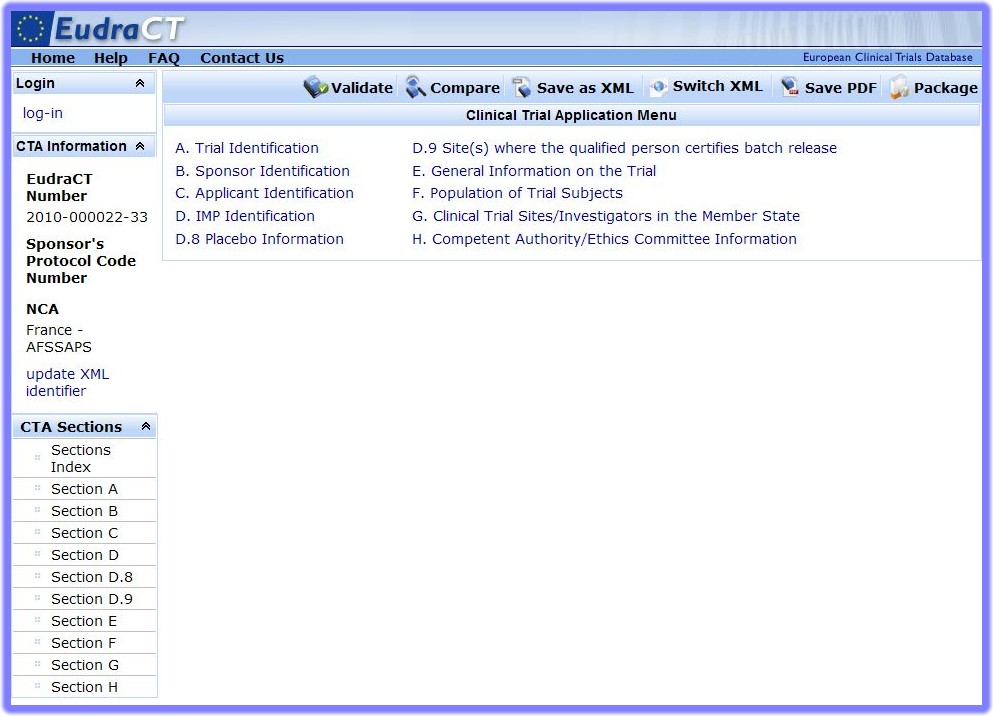
- In the Clinical Trial Application Menu the Task Bar at the top of the screen includes the option 'Compare':

- Click
 to compare the currently loaded CTA with another CTA. The ‘Find XML to Compare to’ page appears:
to compare the currently loaded CTA with another CTA. The ‘Find XML to Compare to’ page appears:
- Click
‘Local File System' to find the XML file you wish to compare with the currently loaded CTA. See "Load an XML file from Local File System" for specific instructions:
Load an XML file from Local File System
Click 'Local File System' and the 'Load an XML file for a Clinical Trial Application' page appears:
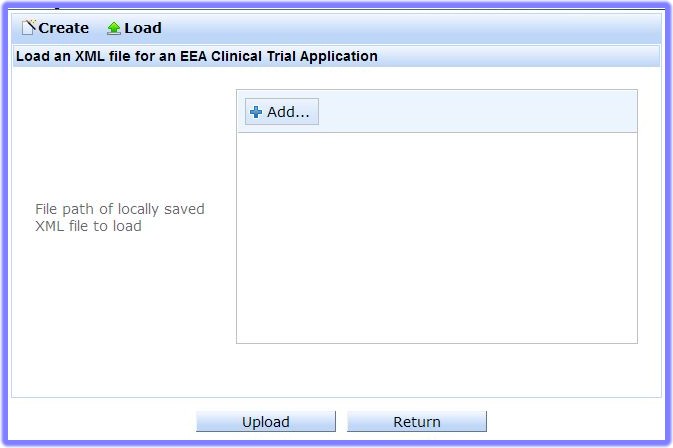
- Now click
 and your local ‘File Upload’ dialogue appears:
and your local ‘File Upload’ dialogue appears:
- Now, find the CTA, which will end with the ‘.xml’ file extension. Once you have found it, click to select it, then click ‘Open’.
- The 'Load an XML file for a Clinical Trial Application' page appears.
- Click ‘Upload’ and the two CTA files undergo a comparison.
When the comparison is complete, the ‘Comparison Results’ page appears:
See Comparison Results section below for more detailed information.
Comparison Results
Note: It is recommended that Internet Explorer 7 is used to ensure the formatting of the report displays correctly. There are known issues surrounding the display of the reports within Firefox.
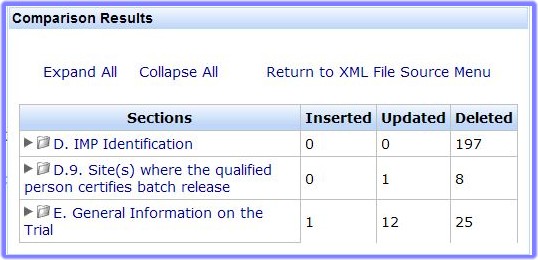
- Click 'Expand All' to see all the differences between the two CTA files or click the arrow beside each section:
- Details of all insertions, updates and deletions.
- Ability to expand and collapse the entire report to the lowest level of granularity .
- Click a section to expand and display child elements.
- Click 'Return to XML File Source Menu' to return to the Clinical Trial Application Menu with the loaded Clinical Trial Application.
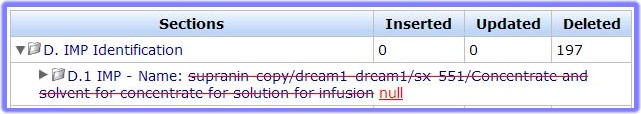
- To view detail of the changes between the two compared files, click on the Section you wish to view in more detail (in this example ‘D. Identification’). The details drop-down beneath the section heading:
- To view the details as tracked changes, scroll down the page.
- The struck through text (replaced) is that which is in the ‘compare’ file
- the underlined text (inserted) is that which is in the loaded file (the CTA currently loaded/being worked on).
Note: It is recommended that Internet Explorer 7 is used to ensure the formatting of the report displays correct. There are known issues surrounding the display of the reports within Firefox.
It is hoped that this functionality will better enable swift comparison of CTAs for both applicants, sponsors and other users of the system. Users are invited to give feedback via the
EudraCT Service Desk
To return to the Clinical Trial Application Menu, click 'Return to XML File Source Menu'.
Now go to the "Clinical Trial Application Menu Overview" for more detailed information on other tasks that can be performed with your Clinical Trial Application.

 Clinical Trial Application or
Clinical Trial Application or  so that you advance to the EudraCT Clinical Trial Application Menu.
so that you advance to the EudraCT Clinical Trial Application Menu.




