Task topic including steps necessary for viewing a Clinical Trial Application draft that you have found using the Search functionality in EudraCTEudraCT (European Union Drug Regulating Authorities Clinical Trials) is the European Clinical Trials Database of all interventional clinical trials of medicinal products commencing in the European Union from 1 May 2004 onwards. The EudraCT database has been established in accordance with Directive 2001/20/EC. SECURE.
This does not result in a copy being made in the ‘edit’ area and there is no possibility of making inadvertent changes. In ‘View’ mode the CTAAcronym: Clinical Trial Application selected is read-only.
 option in the Clinical Trial Application Welcome Page. This is only available if you have logged in to the EudraCT SECURE system as a registered user.
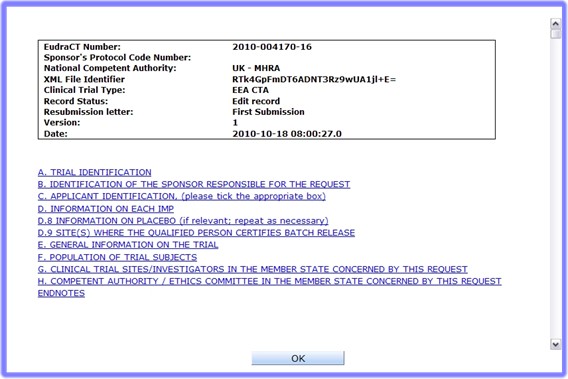
option in the Clinical Trial Application Welcome Page. This is only available if you have logged in to the EudraCT SECURE system as a registered user.View CTA screen

 to exit the viewing functionality and go back to the previous menu.
to exit the viewing functionality and go back to the previous menu.