hyperlink on the left-hand side of the page.
hyperlink on the left-hand side of the page.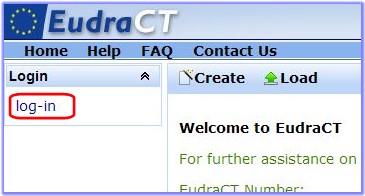
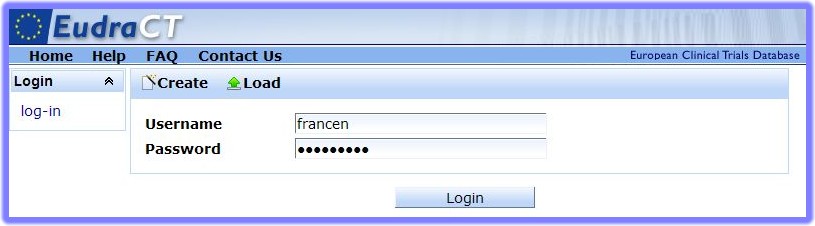
Note:: A full list of the user roles and permissions is available in the "User Roles Overview".
 button.
button.Task including steps describing how to log in to the EudraCTEudraCT (European Union Drug Regulating Authorities Clinical Trials) is the European Clinical Trials Database of all interventional clinical trials of medicinal products commencing in the European Union from 1 May 2004 onwards. The EudraCT database has been established in accordance with Directive 2001/20/EC. system.
 hyperlink on the left-hand side of the page.
hyperlink on the left-hand side of the page.

 button.
button.